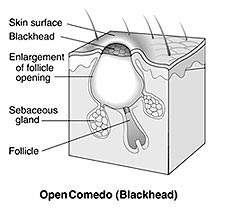
Silica-encapsulated benzoyl peroxide improved acne more with less skin irritation than a conventional form of the medication, according to results of a randomized clinical trial.
Two different strengths of silica-encapsulated drug significantly reduced lesion counts compared with nonencapsulated therapy, Daniela Mavor, of Sol Gel Technologies in Ness Ziona, Israel, reported at the International Congress of Dermatology.
Differences in efficacy and tolerability emerged early and persisted through the study.
“As early as week two, we noted a reduction of acne lesions, which was significantly superior to leading commercial products,” Stanley Shapiro, Ph.D., also of Sol Gel, said in a statement. “At week four, a 75% improvement was achieved.
“Throughout the study, the high-strength acne kits were significantly better tolerated than the two commercial products,” he said.
Benzoyl peroxide is an established therapy for acne, but the compound often causes burning and irritation that ranges from mild to severe.
Sol Gel Technologies has developed a proprietary delivery vehicle consisting of silica microspheres that form a barrier between the skin and the medication inside the spheres.
The microencapsulating process also leads to a continuous release of the active ingredient, Mavor and colleagues explained in a poster presentation.
Investigators evaluated the safety and efficacy of silica-encapsulated benzoyl peroxide in a clinical trial involving 78 patients, ages 16 to 35, with mild or moderate acne vulgaris.
Disease activity required included at least three comedones (noninflammatory lesions), six or more papules or pustules (inflammatory lesions), no more than 25 lesions of either type, and fewer than four nodulocystic acne lesions.
The patients were randomized to one of four treatment groups: 4% or 7% silica-encapsulated benzoyl peroxide, or a 2.5% or 7% commercially available, nonencapsulated formulation of the acne medication.
Study participants were asked to apply assigned medication twice daily for four weeks. Medication came in a kit that included a cleanser, a toner, and a lotion, applied in sequence according to instructions.
The primary outcome measures were change in number, type, and severity of lesions; safety and tolerability; and change in images obtained at each clinic visit.
By week two, the 7% silica-encapsulated treatment had reduced the total lesion count significantly more than either of the two conventional therapies. The percent reduction from baseline also was significantly greater with the high-dose encapsulated formulation (P=0.016 to P=0.033).
Over the four weeks of the study, the 7% encapsulated formulation led to significantly more improvement compared with the two nonencapsulated formulations (P=0.010, P=0.021).
By Investigator Global Assessment, patients using either strength of encapsulated benzoyl peroxide improved by 40% to 50% from week one to week two. In contrast, little change was noted in patients assigned to the conventional acne therapy.
At weeks three and four, patients using the 4% silica-encapsulated formulation had 75% and 82% improvement, respectively, compared with weeks two and three, Mavor reported.
Patients in the 7% silica-encapsulated arm had improvement of 56% and 75% at weeks three and four, respectively.